
As the new energy sector keeps growing, lithium resource development is moving from hype to real projects on the ground. In hard rock lithium ores, spodumene and lepidolite are the two most common and valuable lithium-bearing minerals right now. Both can be used to extract lithium, but they differ in mineral composition, process complexity, and overall project economics. These differences often make or break a project.
Over the past ten-plus years, I've been involved in and visited several spodumene and lepidolite lithium projects. I've seen projects move forward smoothly because the right process route was chosen, and others where early misjudgments led to repeated flow changes and rising costs. Based on that hands-on experience, this article compares the key differences between spodumene and lepidolite in lithium extraction processes. It aims to help project teams spot the core issues when selecting and evaluating routes, and cut down on unnecessary trial-and-error expenses.
Spodumene is a lithium aluminum silicate mineral that typically occurs in pegmatite deposits. Lithium is structurally bound in the crystal lattice, and grades are usually higher. After beneficiation, raw ore Li₂O content is often 1.0% to 2.5%.

In comparison, lepidolite is a lithium-rich mica mineral. It generally has lower lithium oxide content, typically 0.4% to 1.5%, and is often associated with complex gangue minerals like quartz, feldspar, iron-bearing minerals, and other alkali metals such as potassium, rubidium, and cesium.

Here is a quick comparison table I've summarized for spodumene and lepidolite.
Key Properties | Spodumene | Lepidolite |
Mineral Structure | Chain silicate; natural α-spodumene, insoluble in common solvents. | Layered silicate; contains K, F, Al and other accessory elements. |
Li₂O Content | 1.0–2.5% (medium grade) | ~1.0–3.0% |
Core Extraction Requirement | Mandatory high-temperature phase transition (α→β) to improve leachability. | Suitable for chemical decomposition (e.g., acid leaching, salt roasting); no phase transition needed. |
Ore Appearance | Prismatic crystals; colors: white, gray, pink; translucent-transparent. | Scaly/flaky aggregates; colors: lilac, pink, gray, white; pearly luster. |
Typical Occurrence | Major deposits: Australia , Canada , Brazil , China. | Distributed in China, Brazil, Canada, Africa. |
These mineralogical differences directly affect lithium liberation behavior, beneficiation efficiency, and downstream extraction processes:
The core of spodumene processing is high-temperature phase transformation roasting.
The core of lepidolite processing is chemical roasting plus acid leaching, with slightly lower roasting temperatures but higher acid/salt consumption and more complex impurities.
Spodumene Lithium Extraction Process
Spodumene lithium extraction is a well-established industrial route today. Its key step is high-temperature roasting to achieve crystal phase transformation, which greatly boosts lithium's chemical reactivity. Here's a step-by-step look at a typical flow:
1. Beneficiation
Raw spodumene ore goes through crushing and grinding, then enrichment via flotation (direct or reverse flotation) to produce a concentrate with about 5–7% Li₂O, preparing it for downstream extraction.

2. High-Temperature Roasting — The Key "Activation" Step
The enriched spodumene is fed into a high-temperature kiln and roasted at around 1000–1100°C. The crystal structure changes from dense α-phase to porous β-phase (α-LiAlSi₂O₆ → β-LiAlSi₂O₆).
This step uses no chemical reagents—just heat to alter the mineral structure, making it more reactive with acid. It's essentially the "activation" for spodumene extraction.
3. Acid Leaching of Lithium
The roasted β-spodumene is typically acid-roasted with concentrated sulfuric acid at 250–300°C, with a reaction like:
β-LiAlSi₂O₆ + H₂SO₄ → Li₂SO₄ + insoluble alumino-silicate residue
The soluble lithium sulfate is leached into water, moving lithium ions into solution.
4. Purification and Product Preparation
The lithium-bearing solution needs impurity removal (aluminum, iron, magnesium, calcium, etc.) through multiple purification steps to get a cleaner lithium salt solution. Finally, carbonation precipitation (e.g., adding Na₂CO₃ for Li₂CO₃) or other methods (electrolysis, membranes) produce battery-grade lithium carbonate or lithium hydroxide.
Main advantages of the spodumene route:
Proven and mature industrial flowsheet
Relatively stable lithium recovery
Easier impurity control compared to lepidolite
However, high-temperature roasting means higher energy use, and operations need careful control.
Learn more about spodumene beneficiation and processing guide
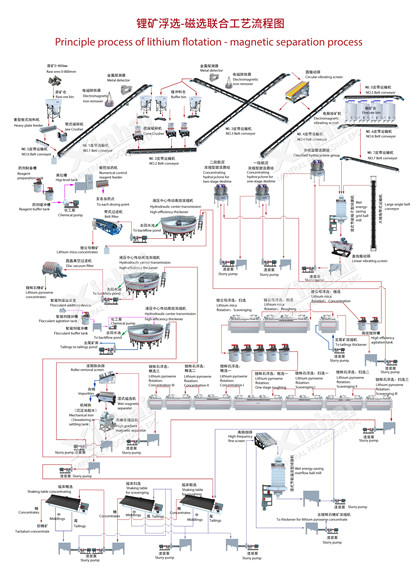
Lepidolite Lithium Extraction Process
Lepidolite lithium extraction is still more technically complex with varied routes. It's less standardized than spodumene and often requires chemical roasting, multi-stage leaching, and deep purification for efficient lithium recovery. Here's an overview of common flows:
1. Beneficiation
Raw ore is crushed and ground, then enriched using flotation or gravity methods to get a concentrate with 2–4% Li₂O, providing feedstock for chemical extraction.
2. Chemical Roasting — The Key Transformation Step
Lepidolite's stable structure needs chemical roasting to break the lattice and release lithium. Common routes include:
Sulfuric acid roasting: Concentrate mixed with concentrated sulfuric acid and roasted at 250–350°C or higher, converting lithium and potassium to sulfates like soluble Li₂SO₄.
Sulfate/lime salt roasting: e.g., Na₂SO₄–CaO system at 800–900°C, using solid-phase reactions to change lithium form for easier leaching.
Chlorination roasting or lime roasting: e.g., mixed with CaCl₂, converting lithium to water-soluble chloride for further leaching.
Overall, lepidolite roasting involves adding acids or salts—not just structural change but chemical reactions. This differs markedly from spodumene's pure thermal transformation.

3. Leaching
Roasted material is leached with water or dilute acid, moving lithium into solution as Li⁺ or Li₂SO₄. But impurities like potassium, sodium, aluminum, and fluorine also dissolve heavily, making the leach liquor complex and increasing downstream purification load.
4. Deep Purification and Lithium Salt Preparation
High impurity levels (especially K, Na, Al, F) require multi-step removal, such as staged precipitation for aluminum, chemical defluorination, or ion exchange for alkali metals. The cleaned lithium solution then goes to carbonation precipitation for battery-grade lithium carbonate or hydroxide.
While lepidolite ores can have advantages in some geological settings, the extraction process typically features:
Greater sensitivity to ore variations
More complex flowsheet design
Higher demands on waste and impurity handling
So lepidolite-based projects usually need more detailed metallurgical testing before route selection.
Learn more about lepidolite beneficiation and processing guide
From a technical view, the differences between lepidolite and spodumene can be summed up as:
Process complexity: Spodumene flows are generally simpler and more standardized, while lepidolite involves more unit operations.
Energy use: Both need thermal treatment, but lepidolite routes often combine multiple heating and chemical steps.
Lithium recovery stability: Spodumene projects tend to achieve more stable and predictable recoveries.
Impurity control: Co-existing alkali metals make lepidolite extraction more challenging.
Environmental and operational considerations: Both produce tailings and residues, but lepidolite's higher impurity removal needs often lead to more complex waste streams.
Spodumene and lepidolite both play vital roles in the lithium industry, but their differences in composition, processing, and economics define their respective places. Spodumene has the edge in efficiency, while lepidolite offers strategic flexibility.

Conclusion
No single lithium extraction process fits all hard rock ores. The lepidolite vs spodumene comparison clearly shows that ore characteristics fundamentally drive the process route, technical risks, and economic outcomes.For project owners, investing in proper ore testing and process evaluation early on can greatly reduce downstream uncertainties and avoid costly operational adjustments.
If you're evaluating a lepidolite or spodumene lithium project, feel free to contact us!
To find out more about our products and solutions, please fill out the form below and one of our experts will get back to you shortly.